Abstract
Background
Platelets, harvested for transfusion, have a relatively short "shelf life" of 5 days. With increasing storage, platelets develop structural and functional changes called "platelet storage lesions". These changes result not only in reduced hemostatic response to agonists but also in an accelerated clearance from the circulation in vivo. The precise mechanisms of clearance of platelets aged in vitro (or vivo) are not known. There is an increased exposure of the anionic phospholipid phosphatidylserine (PS) on the cell surface of stored platelets. PS is a well-known tag for clearance of cells from the circulation by the macrophage PS receptors. PS-expressing stored platelets are also rapidly cleared from the circulation, presumably through the similar mechanism. Targeting this pathway could improve the recovery of stored platelets following transfusion by inhibiting storage-induced PS exposure
Results and Discussion
Actin is a major PS binding proteins in platelets. In resting platelets, actin filaments are at the periphery and in close proximity to the membrane. We hypothesize that the interaction with actin filaments constrains the mobility of PS in the inner leaflet. We generated artificial bilamellar liposomes mimicking the intra (PS) or extra (PC) cellular phospholipid composition of the membrane bilayer. When these liposomes were incubated with platelet lysate, the most striking enrichment was seen in actin, which was increased 5-fold in PS-containing liposome compared to control PC liposomes. These data suggest that actin and actin-associated proteins may, in fact, be the major inner leaflet PS-binding proteins in resting platelets.
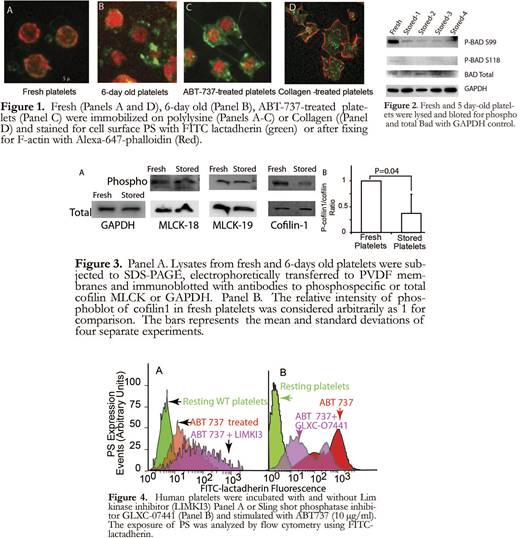
Cortical actin retracts from the membrane with PS exposure in senescent platelets: We examined the morphological changes in resting fresh platelets, five-day-old platelets and ABT-737 (10 μM) treated platelets by immunofluorescence microscopy. Platelets immobilized on polylysine-coated plates were immunostained with FITC-lactadherin and Alexa- 647-phalloidin. As shown in Figure 1, resting platelets do not express PS (no or minimal FITC-lactadherin staining). Actin filaments (Alexa-647-phalloidin) are distributed in the subcortical region as a thin submembranous network. In 6-day old stored platelets, there is significant increase in platelet surface FITC-lactadherin staining. In addition, in most platelets actin filaments have retracted from the subcortical region towards the center of the platelet (compare figure 1 panels A and B
Bad phosphorylation is significantly diminished in stored platelets. Phosphorylated Bad is sequestered in the cytosol by binding to the scaffolding protein 14-3-3. Furthermore, phosphorylation of S118 inhibits apoptosis by preventing its association with Bcl-xL. Dephosphorylation of Bad may initiate intrinsic apoptosis by associating with Bcl-xL. We determined the phosphorylation status of Bad in stored platelets by Phosphoproteomic and WB analyses of stored and fresh human platelets ( Figure-2) Both methods showed a drastic decrease in Bad is phosphorylation at serine residues 99 and 118 in stored platelets.
Activation of cofilin-1 by dephosphorylation in stored platelets. Like Bad, phosphorylation of cofilin-1 is also markedly decrease in stored platelets as shown by WB analyses (Figure-3). It is unclear how the apoptotic pathways lead to cofilin-1 activation (dephosphorylation). Caspases are known to activate slingshot phosphatase which could then activate cofilina1 by its dephosphorylation. LIM- kinases phosphorylate and, thus, inactivate cofilin-1 whereas slingshot phosphatases dephosphorylate and activate cofilin-1. Our data show that the Lim kinase inhibitor, as expected increased ABT-737-induced PS exposure on platelets (Figure 4A), whereas slingshot phosphatase inhibitor inhibited ABT-737-induced PS exposure at a 1-μM concentration (Figure 4B)
Conclusion
Our data show that activation of apoptotic pathways is responsible for activation of cofilin and subsequent PS exposure by actin reorganization in stored platelets. Targeting this pathway could improve the recovery of stored platelets following transfusion by inhibiting storage-induced PS exposure.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal